INTRODUCTION
Introducing LIMS
Laboratories, in today’s transitional healthcare environment, are increasingly facing operational challenges in managing their workflow, data, and security. Countless hours are lost on tedious administrative tasks such as manual data entry, tracking sample locations, and monitoring regulatory compliance. The risk of unauthorized access, data breach, accidental mishandling, or accidental deletion raises a perpetual data security concern.
Laboratory Information Management System (LIMS) is designed to tackle these key issues. The software’s powerful automation capabilities streamline the entire process of laboratory management. From sample monitoring and inventory management to data collection and analysis, LIMS transforms laboratory operations by eliminating manual errors, increasing efficiency, and ensuring data integrity.
DEFINITION
What is a LIMS?
LIMS is a software designed to address the operational requirements of contemporary laboratories. It automates and streamlines various laboratory processes and data administration and assists in organizing workflow. The system functions as a central repository for storing, managing, and analyzing laboratory data, such as sample monitoring, test results, quality control, and instrument integration. It also improves productivity, efficiency, and accuracy by decreasing manual errors, minimizing paperwork, and eliminating redundant duties.
LIMS is essential for optimizing laboratory operations, fostering collaboration, and facilitating efficient data administration and analysis.
BENEFITS
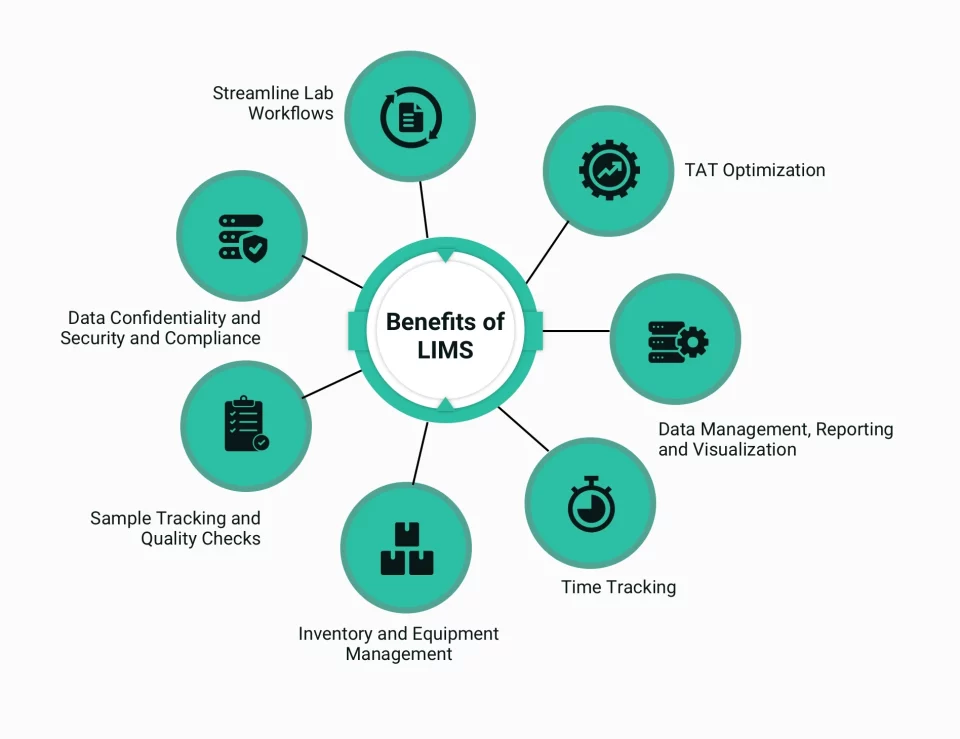
Advantages of using laboratory information management system
 LIMS streamlines laboratory workflows and maximizes resource utilization
LIMS streamlines laboratory workflows and maximizes resource utilization
Accuracy, efficiency, and data integrity are one of the prime priorities for laboratories today. LIMS facilitates a holistic integration of these elements while providing numerous other advantages. The following are a few of the most significant advantages of using a LIMS.
Streamlining laboratory workflows
LIMS is designed to expedite laboratory workflows by automating and optimizing integral processes. By automating tasks, enhancing data management, and maximizing resource utilization, the software streamlines laboratory workflows. It allows laboratories to operate more effectively, increases productivity, and focuses on delivering accurate and timely results.
Turnaround time optimization
LIMS optimizes the turnaround time for diagnostic tests due to its real-time overview of the testing procedure. It automates multiple testing stages, such as sample preparation, data analysis, and report generation. Thereby decreasing the time required to complete each test.
Data confidentiality, security, and compliance
Integrating a robust LIMS not only assists in centralizing data storage but also automates security protocols and incorporates access controls, encryption protocols, and user authentication mechanisms, preventing unauthorized access into the system. Moreover, it provides extensive audit traces, enabling a transparent and traceable record of all data activities.
With LIMS, a laboratory can adhere to various regulations with real-time quality control and monitoring. It is possible to prepare, record, and inspect samples to ensure regulatory compliance. Specimens that do not satisfy the criteria can be reported automatically.
Data management, reporting, and visualization
Laboratories frequently contend with data management, precision, time constraints, technical customization, result interpretation, and regulatory reporting compliance. LIMS can capture and store laboratory data efficiently, making it readily accessible for reporting purposes. It can also automate the report generation process, greatly reducing the time and effort required to generate customized reports for a laboratory’s specific needs.
LIMS provides sophisticated tools and visualization capabilities for data analysis and generates detailed, actionable reports to aid decision-making.
Sample tracking and quality checks
Inadequate sample management always results in delayed clinical reporting or missing samples. Depending on the type of laboratory, LIMS performs thorough quality checks on samples or final products. It also automates the logging of samples.
Inventory and equipment management
Complicated inventory management requires a significant amount of time and concentration and can be a cumbersome task for laboratory administrators. LIMS, in addition to measuring, recording, and controlling inventories, can also manage laboratory equipment. The software equips operators with the capability to schedule essential maintenance actions and instrument calibrations. LIMS also permits laboratory technicians to continuously monitor the technical condition of the equipment.
Time tracking
LIMS' time-monitoring feature enables the tracking and documentation of the time spent on tasks such as sample processing, data entry, analysis, instrument maintenance, and quality assurance procedures. This feature provides valuable insights into the productivity and efficacy of laboratory operations by identifying bottlenecks and effectively allocating resources.
KEY CONSIDERATIONS
What to consider when developing LIMS?
Each LIMS has its strengths and weaknesses. Regardless of the type of solution chosen, it is essential to ensure that it is adaptable, scalable, and dependable to accommodate changing requirements.
Some key considerations when choosing a LIMS include:
Customized needs of the laboratory: The functionality of a LIMS needs to be assessed to ensure that it aligns with the specific needs of the laboratory. The solution should allow for easy configuration and workflow customization.
Integration capabilities: The LIMS under consideration should have the ability to integrate with other laboratory instruments, systems, and data sources. Seamless integration allows for data exchange, instrument control, and streamlined workflows. Compatibility with various data formats and instruments is important for efficient data management.
Data security and compliance: One needs to ensure that the chosen LIMS provides robust data security measures, including user access controls, encryption, and audit trail capabilities. It should also comply with relevant regulatory standards and guidelines, such as the Health Insurance Portability and Accountability Act, General Data Protection Regulation, or Food and Drug Administration regulations, depending on the laboratory's requirements.
Cost and scalability: The total cost of ownership, including initial investment, maintenance fees, and any additional costs associated with customization or integration, needs to be considered. In addition, the scalability of the LIMS to accommodate potential growth or changing laboratory needs in the future should be evaluated.
The adoption of LIMS unlocks a world of possibilities, allowing laboratories to focus on innovation, breakthrough discoveries, and advancing scientific knowledge.
THE FUTURE
The potential for LIMS is limitless
The future of LIMS holds enormous potential for laboratories around the globe. As more organizations recognize the need for automated laboratory data management solutions, with LIMS at the center of a completely integrated digital ecosystem, this industry is anticipated to experience rapid growth in the near future. In the 21st century, the demand for more robust healthcare data capabilities will only increase, and a modern LIMS must be adaptable enough to balance the requirements for data accessibility, data integrity, and cybersecurity.
WE CAN HELP
Develop an intelligent laboratory information management system with accuracy, efficiency, and data integrity
Asahi Technologies is a proven healthcare technology solutions provider. Combining our full-stack development expertise with domain knowledge, we deliver industry-specific applications that solve complex health technology challenges.
We guide you to reimagine your strategies, unlock resources, and improve your capabilities to succeed in the face of rapid technological changes. Healthcare is undergoing a massive transformation, and we know you need actionable and evidence-based insights to plan your future moves. Risk assessments, compliance reviews, continuous learning, and competitive intelligence keep us agile and prepared. We leverage technology trends to help clients conquer challenges in their digital transformation efforts.
We are problem solvers, solution builders, and trusted partners.






